|
Bone regeneration remains a major clinical challenge, especially when infection necessitates prolonged antibiotic treatment. This study presents a membrane composed of self-assembled and interpenetrating GL13K, an antimicrobial peptide (AMP) derived from a salivary protein, in a collagen membrane for antimicrobial activity and enhanced bone regeneration. Commercially available collagen membranes were immersed in GL13K solution, and self-assembly was initiated by raising the solution pH to synthesize the multifunctional membrane called COL-GL. Incorporation of GL13K led to antimicrobial and anti-fouling activity against early oral surface colonizer Streptococcus gordonii while not affecting fibroblast cytocompatibility or pre-osteoblast osteogenic differentiation. GL13K in solution also reduced macrophage inflammatory cytokine expression and increased pro-healing cytokine expression. Bone formation in a rat calvarial model was accelerated at eight weeks with COL-GL compared to the gold-standard collagen membrane based on microcomputed tomography and histology.
International Journal of Biological Macromolecules, 2024. Link here |
|
Preventing bacterial infection and promoting osseointegration are essential for the long-term success of titanium (Ti) implants. In this study, we developed a multifunctional nanocoating on Ti mini-implants to simultaneously address these challenges. The nanocoating consists of self-assembled antimicrobial peptides GL13K and silver nanoparticles, referred to as Ag-GL. Our results showed that the Ag-GL coating did not alter the surface morphology of the mini-implants. Ag-GL coated mini-implants demonstrated a two orders of magnitude reduction in colony-forming unit (CFU) values compared to the noncoated eTi group, resulting in minimal inflammation and no apparent bone destruction in a bacterial infection in vivo model. When evaluating osseointegration properties, micro-CT analysis, histomorphometric analysis, and pull-out tests revealed that the Ag-GL coating significantly enhanced osseointegration and promoted new bone formation in vivo.
Colloids and Surfaces B: Biointerfaces, 2023. Link here |
|
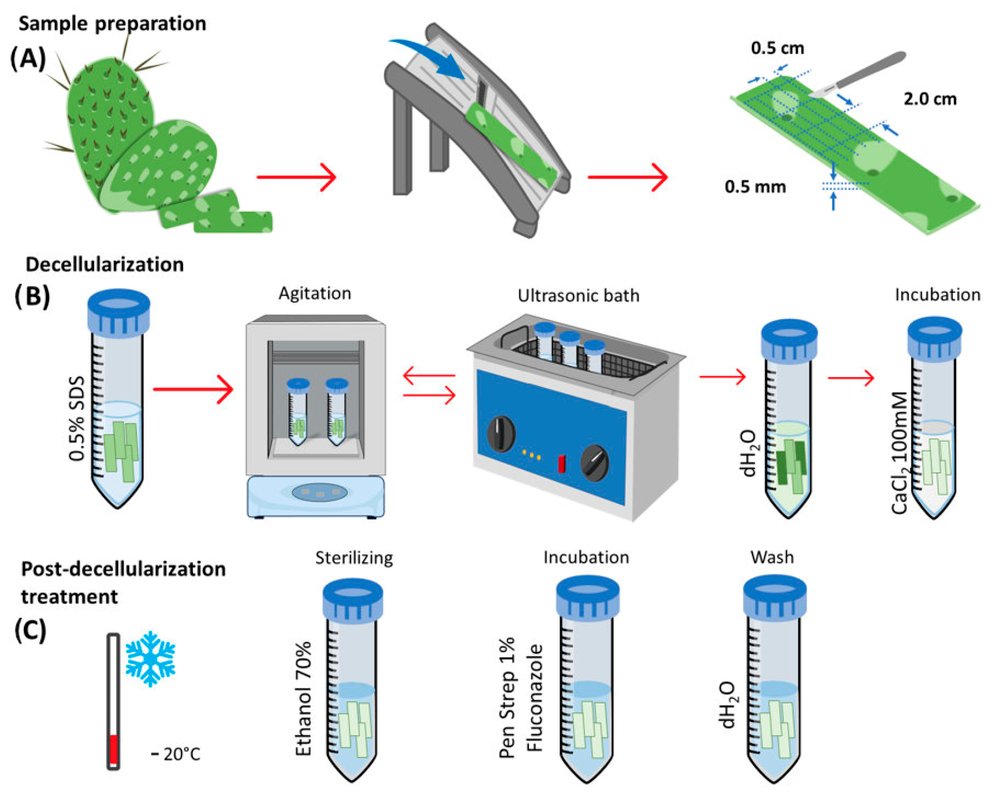
Opuntia Ficus-indica, or nopal, is traditionally used for its medicinal properties in Mexico. This study aims to decellularize and characterize nopal (Opuntia Ficus-indica) scaffolds, assess their degradation and the proliferation of hDPSC, and determine potential pro-inflammatory effects by assessing the expression of cyclooxygenase 1 and 2 (COX-1 and 2). This study demonstrates the potential application of nopal scaffolds in tissue engineering and regenerative medicine or dentistry, owing to their structural characteristics, degradation properties, mechanical properties, ability to induce cell proliferation, and lack of enhancement of pro inflammatory cytokines.
Journal of Functional Biomaterials, 2023. Link here |
|
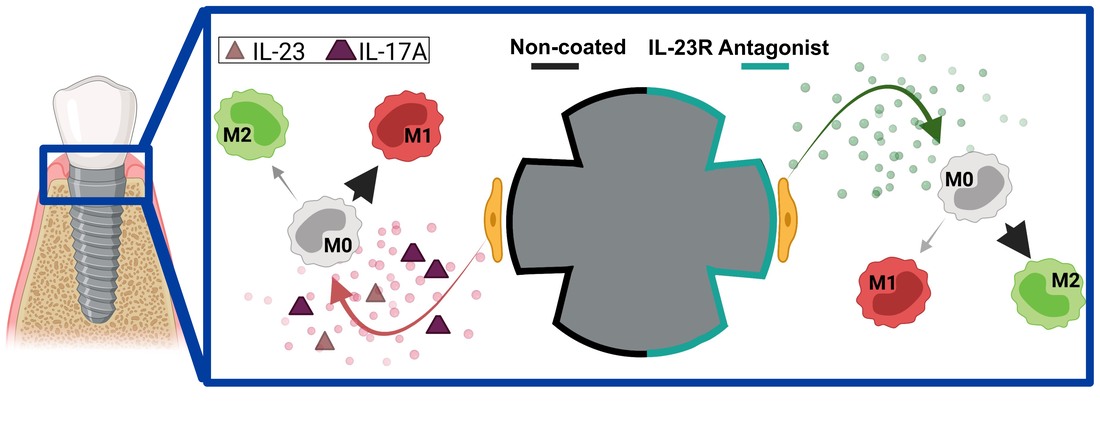
Peri-implantitis, caused by an inflammatory response to pathogens, is the leading cause of dental implant failure. Poor soft tissue healing surrounding implants – caused by inadequate surface properties – leads to infection, inflammation, and dysregulated keratinocyte and macrophage function. One activated inflammatory response, active around peri-implantitis compared to healthy sites, is the IL-23/IL-17A cytokine axis. Implant surfaces can be synthesized with peptide nanocoatings to present immunomodulatory motifs to target peri-implant keratinocytes to control macrophage polarization and regulate inflammatory axises toward enhancing soft tissue healing. Our results support development of IL-23R noncompetitive antagonist nanocoatings to reduce the pro-inflammatory IL-23/17A pathway and augment macrophage polarization toward a pro-regenerative phenotype.
Dental Materials, 2023. Link here |
|
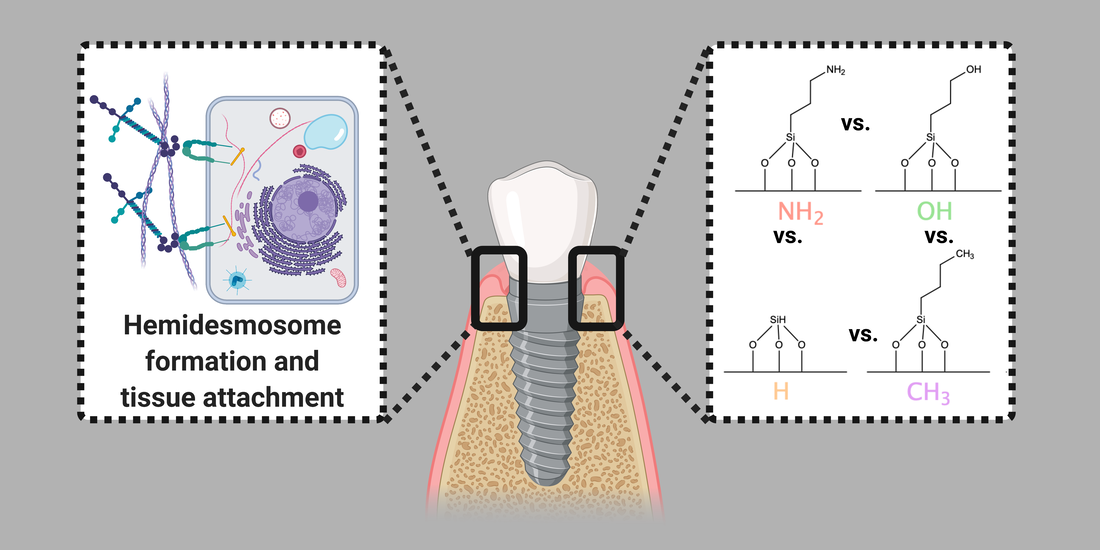
Previous studies have shown hydrophilic/hydrophobic implant surfaces stimulate/ hinder osseointegration. An analogous concept was applied here using common biological functional groups on a model surface to promote oral keratinocytes (OKs) proliferation and hemidesmosomes (HD) to extend implant lifespans through increased soft tissue attachment. However, it is unclear what physicochemistry stimulates HDs. Modification with the OH chemical group showed the highest OKs proliferation and HD expression. The OKs response on OH surfaces appeared to not correlate to the amount or thickness of adsorbed model proteins.
Journal of Biomedical Materials Research: Part A, 2022. Link here |
|
The purpose of this study was to demonstrate the likelihood of the polyetheretherketone (PEEK), zirconia (ZrO2) and titanium (Ti) discs to support proliferation and hemidesmosome formation of gingival cells. The WCA ranged from 70.2 (Ti) to a maximum of hydrophobicity of 93.3 (PEEK). Ra was highest on ZrO2, followed by PEEK. Ti showed the most keratinocyte metabolic activity at 1, 3 and 5 culture periods. Contrarily, ZrO2 and PEEK discs had lower keratinocytes metabolic activity in all observation times, with no statistical differences between both groups. Integrin α6 and β4 expression was highest on TCPS and ZrO2 compared to Ti and PEEK. Keratinocytes proliferated faster on Ti than on ZrO2 and PEEK substrates, and expression of hemidesmosome formation markers, integrin α6 and β4, were higher on ZrO2 than either Ti or PEEK.
The International Journal of Oral and Maxillofacial Implants, 2023. Link here |
|
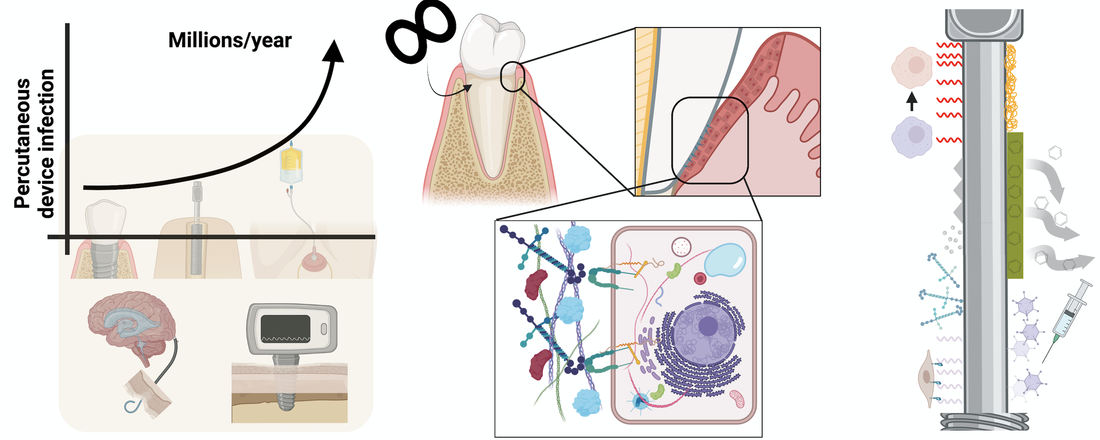
The percutaneous device dilemma describes etiological factors, centered around the disrupted epithelial tissue surrounding non-remodelable devices, that contribute to rampant percutaneous device infection. Natural percutaneous organs, in particular their extracellular matrix mediating the “device”/epithelium interface, serve as exquisite examples to inspire longer lasting long-term percutaneous device design. For example, the tooth’s imperviousness to infection is mediated by the epithelium directly surrounding it, the junctional epithelium (JE).Here, the authors survey the multifaceted functions of the JE, emphasizing the role of the matrix, with a particular focus on hemidesmosomes and their five main components. The authors highlight the known (and unknown) effects dental implant – as a model percutaneous device – placement has on JE regeneration and synthesize this information for application to other percutaneous devices. The authors conclude with a summary of bioengineering strategies aimed at solving the percutaneous device dilemma and invigorating greater collaboration between clinicians, bioengineers, and matrix biologists.
Bioactive Materials, 2022. Link here |
|
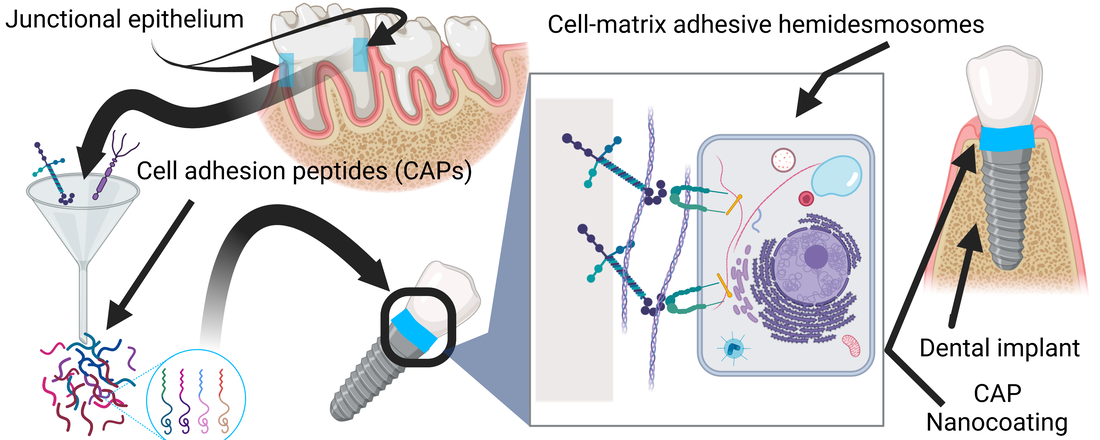
Teeth, long-lasting percutaneous organs, feature soft tissue attachment through adhesive structures, hemidesmosomes, in the junctional epithelium basement membrane adjacent to teeth. This soft tissue attachment prevents bacterial infection of the tooth despite the rich – and harsh – microbial composition of the oral cavity. Conversely, millions of percutaneous devices (catheters, dental, and orthopedic implants) fail from infection yearly. Standard of care antibiotic usage fuels antimicrobial resistance and is frequently ineffective. Infection prevention strategies, like for dental implants, have failed in generating durable soft tissue adhesion – like that seen with the tooth – to prevent bacterial colonization at the tissue-device interface. Here, inspired by the impervious natural attachment of the junctional epithelium to teeth, we synthesized four cell adhesion peptide (CAPs) nanocoatings, derived from basement membranes, to promote percutaneous device soft tissue attachment.
Acta Biomaterialia, 2021. Link here |
|
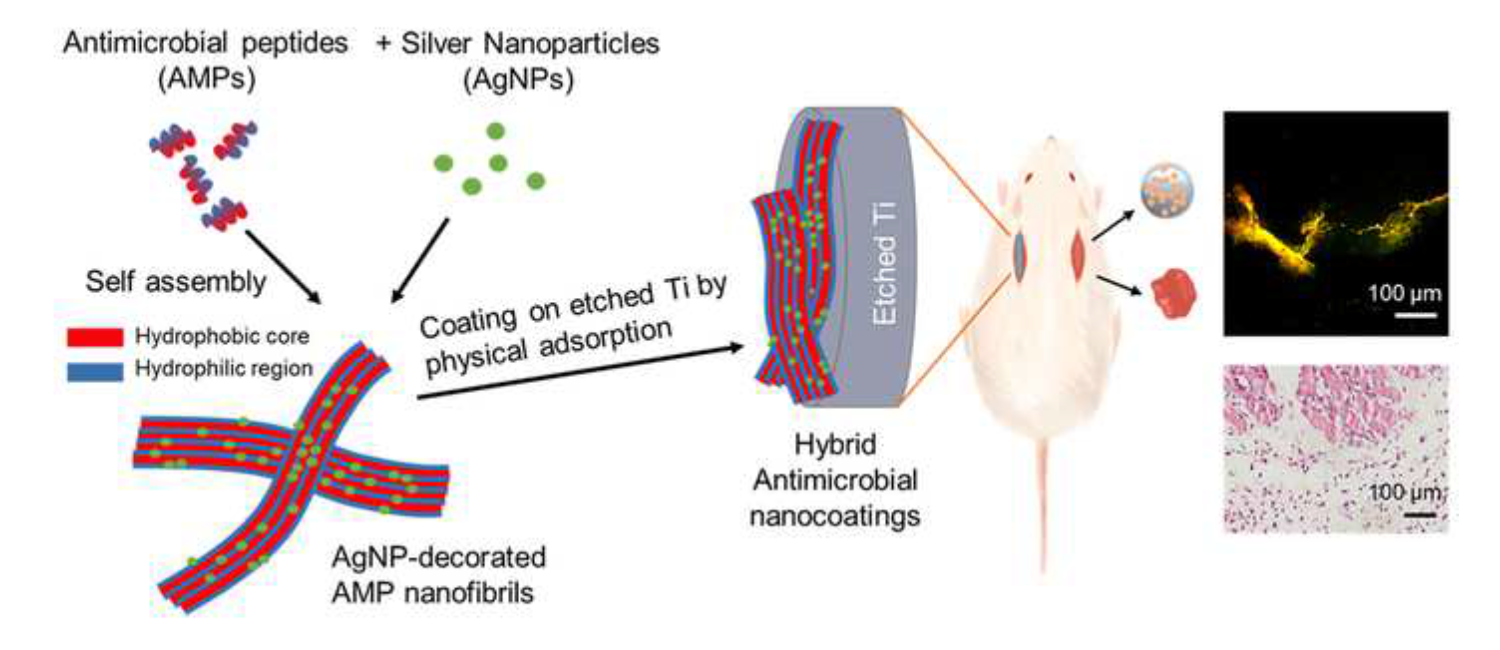
Antimicrobial coatings are one of the most promising strategies to prevent bacterial infections in orthopedic and dental implants. Combining antimicrobial agents with different antimicrobial mechanisms might have synergistic effects and be more potent. In this work, we synthesized self-assembled antimicrobial amphiphiles of an AMP, GL13K. Then, we decorated the AMP nanostructures with AgNPs, which were finally used to coat etched Ti (eTi) surfaces. The strong hydrogen bonding between the AMP amphiphiles and the polar eTi yielded a robust and stable coating. The hybrid coating showed relevant antimicrobial activity in an in vivo subcutaneous infection model in rats. This work advances the application of AgNP/AMP nanocomposites as effective coatings for prevention of implant infections.
Acta Biomaterialia, 2021. Link here |
|
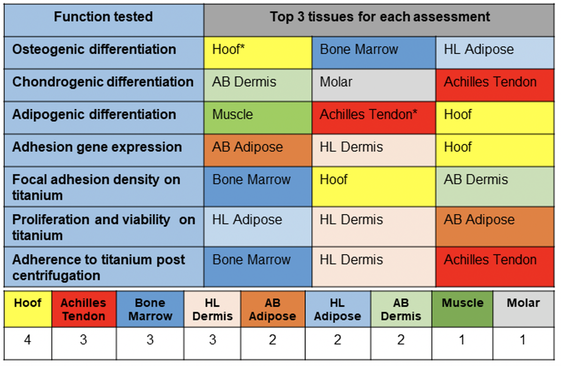
Transdermal osseointegrated prosthesis have relatively high infection rates leading to implant revision or failure. A principal cause for this complication is the absence of a durable impervious biomechanical seal at the interface of the hard structure (implant) and adjacent soft tissues. This study explores the possibility of recapitulating an analogous cellular musculoskeletal-connective tissue interface, which is present at naturally occurring integumentary tissues where a hard structure exits the skin, such as the nail bed, hoof, and tooth. Hoof-associated superficial flexor tendon and Achilles tendon ranked the highest in both differentiation and adhesion assessments. These findings support further preclinical research of these tissue specific-derived MSCs in vivo in a transdermal osseointegration implant model.
Stem Cell Research & Therapy, 2021. Link here |
|
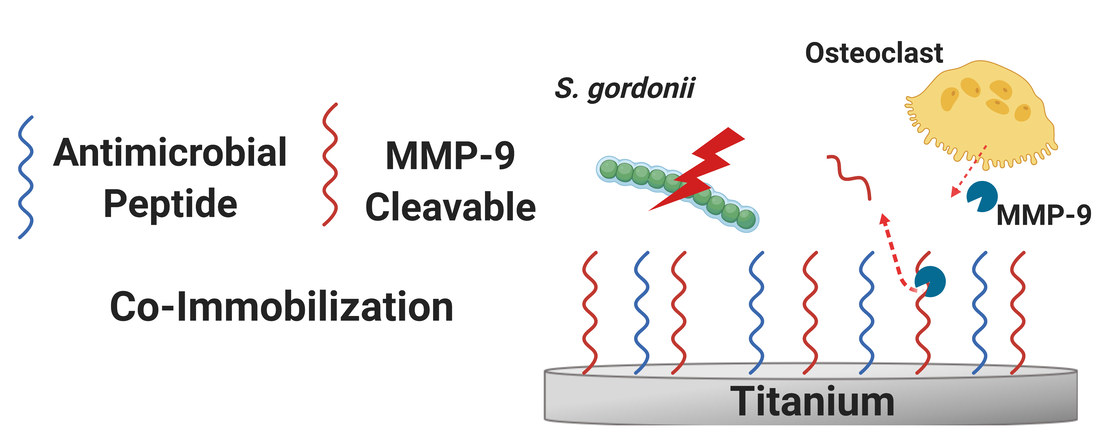
Functionalization of dental and orthopaedic implants with multiple bioactivities is desirable to obtain surfaces with improved biological performance and reduced infection rates. While many approaches have been explored to date, nearly all functionalized surfaces are static, i.e., non-responsive to biological cues. However, tissue remodeling necessary for implant integration features an ever-changing milieu of cells that demands a responsive biomaterial surface for temporal synchronization of interactions between biomaterial and tissue. Here, we successfully synthesized a multi-functional, dynamic coating on titanium by co-immobilizing GL13K antimicrobial peptide and an MMP-9 – a matrix metalloproteinase secreted by bone-remodelling osteoclasts – responsive peptide.
Materials Science and Engineering: C, 2021. Link here |
|
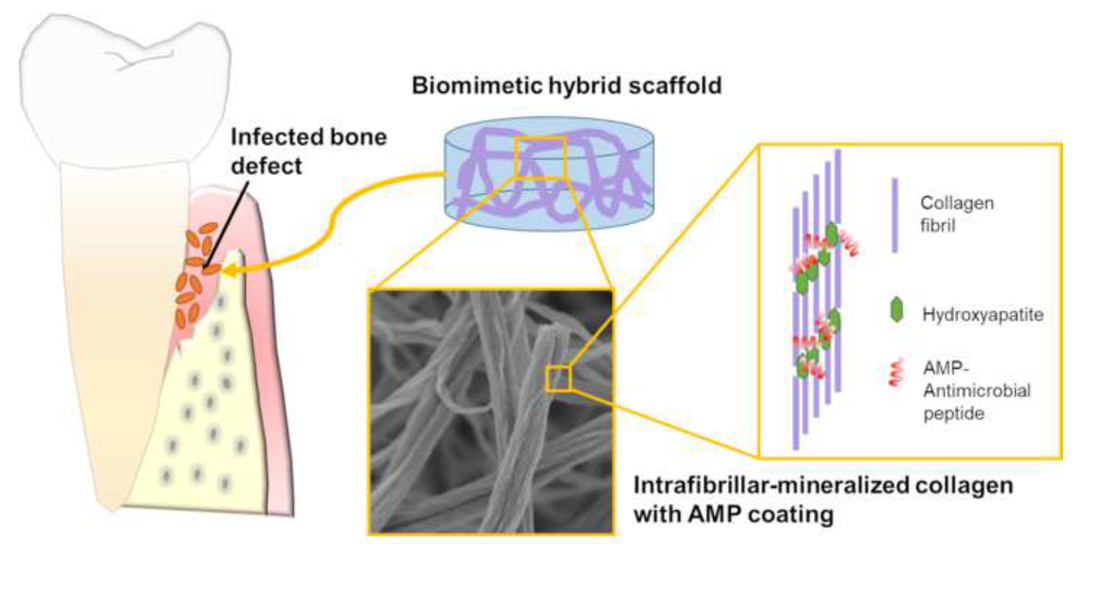
Infection in hard tissue regeneration is a clinically-relevant challenge. Development of scaffolds with dual function for promoting bone/dental tissue growth and preventing bacterial infections is a critical need in the field. Here we fabricated hybrid scaffolds by intrafibrillar-mineralization of collagen using a biomimetic process and subsequently coating the scaffold with an antimicrobial designer peptide with cationic and amphipathic properties. The highly hydrophilic mineralized collagen scaffolds provided an ideal substrate to form a dense and stable coating of the antimicrobial peptides. The process of scaffold fabrication is versatile and can be used to control mineral load and/or intrafibrillar mineralized scaffolds made of other biopolymers.
Bioactive Materials, 2021. Link here |
|
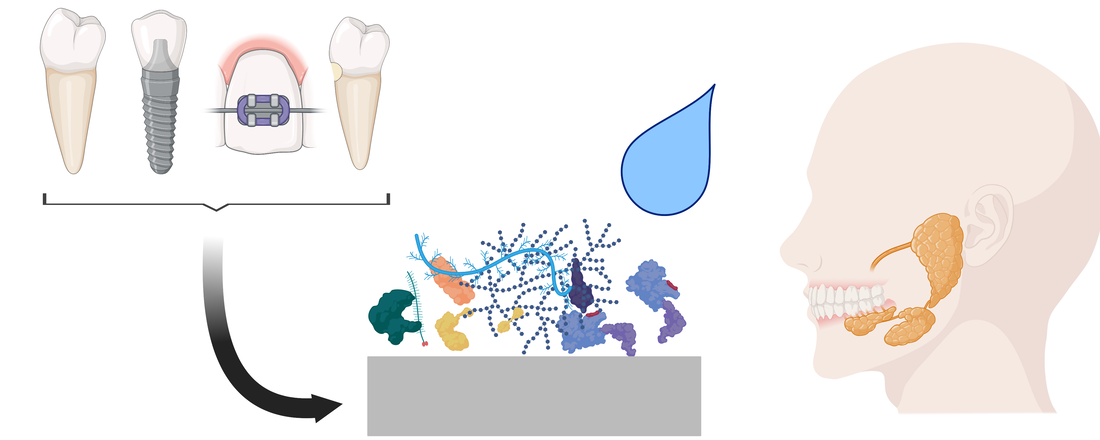
The salivary pellicle, an adlayer formed by adsorption of salivary components on teeth and dental biomaterials, has direct consequences on basic outcomes of dentistry. Here, we provide an overview of salivary pellicle formation processes with a critical focus on dental biomaterials. We survey the many effects salivary pellicles have on dental biomaterials and highlight its implications on design criteria for dental biomaterials. Future investigations may lead to rationally designed dental biomaterials to control the salivary pellicle and enhance material function and patient outcomes.
Colloids and Surfaces B: Biointerfaces, 2021. Link here |
|
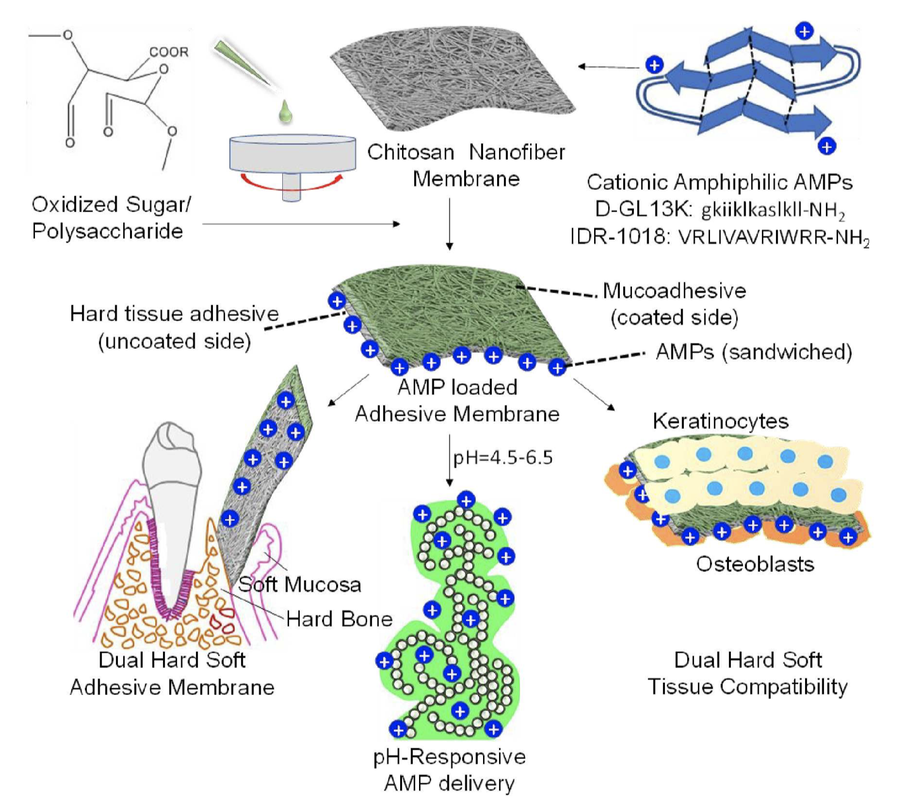
Bio-adhesive membranes with controllable and reversible under water adhesion are desirable for several biomedical applications ranging from bio-sensing, drug/ therapeutic delivery and tissue regeneration. Here, we present dual soft mucosal and hard bone/ enamel tissue adhesive nanofiber membranes composed of chitosan and pectin derivatives for pH-controlled delivery of antimicrobial peptides (AMPs) in the oral cavity. We envision these membranes to function as adhesive gingival grafts and guided bone regeneration (GBR) membranes at the hard-soft tissue interface, while simultaneously protecting against oral infections.
Biomacromolecules, 2020. Link here |
|
Dental clinicians have relied for centuries on traditional dental materials (polymers, ceramics, metals, and composites) to restore oral health and function to patients. Clinical outcomes for many crucial dental therapies remain poor despite many decades of intense research on these materials. Recent attention has been paid to biomolecules as a chassis for engineered preventive, restorative, and regenerative approaches in dentistry.
In this review, we survey the range of biomolecules that have been used across dental biomaterials. Our particular focus is on the key biological activity imparted by each biomolecule toward prevention of dental and oral diseases as well as restoration of oral health. We conclude our narrative review with an outlook on the future of biomolecules in dental biomaterials and potential avenues of innovation for biomaterial-based patient oral care. Journal of Materials Chemistry B, 2020. Link here Cover art selected for the front cover! |
|
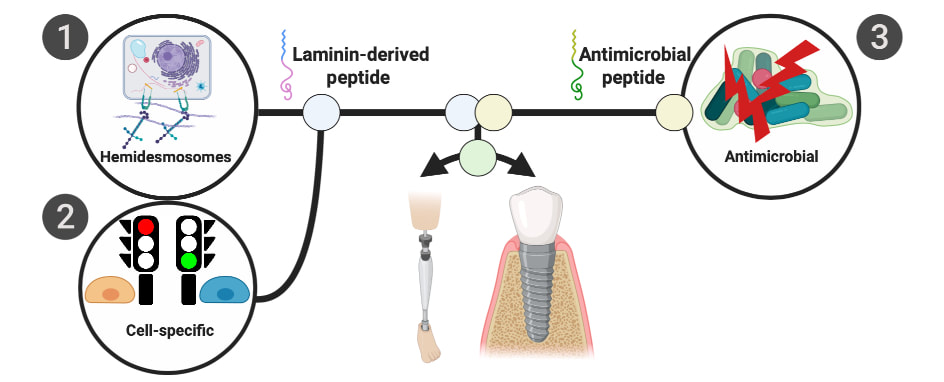
Percutaneous devices like orthopedic prosthetic implants for amputees, catheters, and dental implants suffer from high infection rates. A critical aspect mediating peri-implant infection of dental implants is lack of a structural barrier between the soft tissue and implant surface which could impede bacteria access and colonization of exposed implant surfaces. In response to this healthcare burden, a simultaneously hemidesmosome-inducing antimicrobial multifunctional implant surface was engineered. A designer antimicrobial peptide, GL13K, and a laminin-derived peptide, LamLG3, were co-immobilized with two different surface fractional areas. Overall, these multifunctional surfaces may be able to reduce peri-implantitis rates and enhance the success rates of all percutaneous devices via strong antimicrobial activity and enhanced soft tissue attachment to implants.
ACS Biomaterials Science and Engineering, 2020. Link here |
|
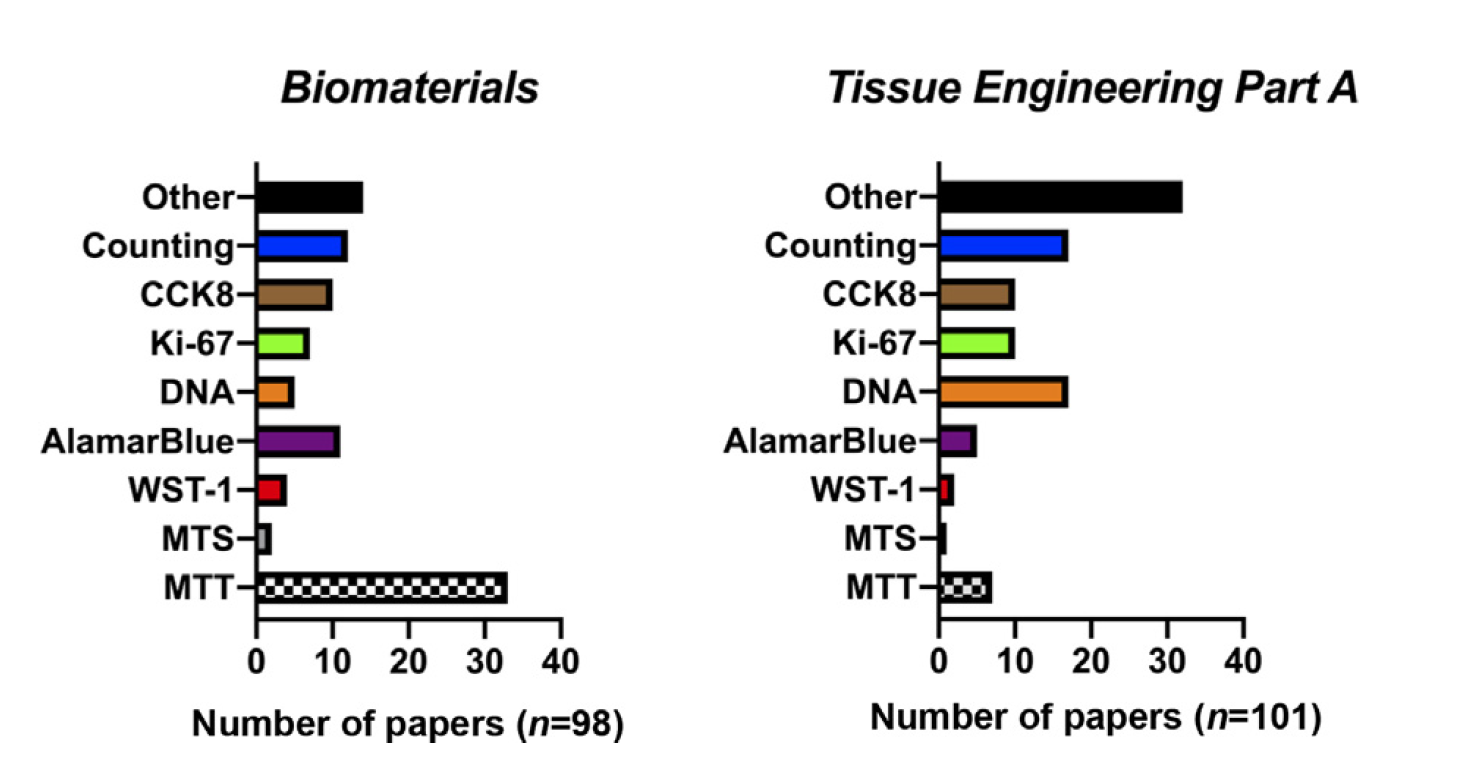
Proliferation is a fundamental cell behavior integral to biomaterial and tissue engineering outcomes. Numerous methods have been developed in order to quantify this relatively complex cell behavior. Measuring proliferation in the context of biomaterials and tissue engineering poses unique challenges. Seemingly trivial factors can affect measurement outcomes and make interpretation challenging. This chapter outlines the array of methods used to measure proliferation and factors to consider when performing these experiments. In particular, we focus on factors relevant to the fields of biomaterials and tissue engineering and criticize each measurement method. We intend this chapter to be a primer for those working in the field to help improve scientific use and interpretation of these seemingly simple cell proliferation measurements.
On the proliferation of cell proliferation tests, in Handbook of Biomaterials Biocompatibility, 2020 Link here |
|
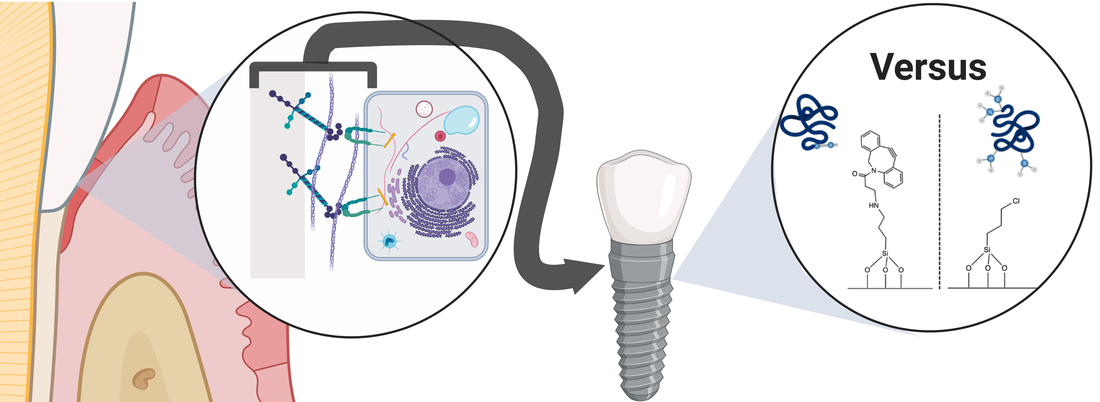
Many chemical routes have been proposed to immobilize peptides on biomedical device surfaces, and in particular, on dental implants to prevent peri-implantitis. While a number of factors affect peptide immobilization quality, an easily controllable factor is the chemistry used to immobilize peptides. These factors affect peptide chemoselectivity, orientation, etc. and ultimately affect biological activity. Our results highlight that the most effective immobilization chemistry for optimal peptide activity is dependent on the specific system (substrate/peptide/cell/biological activity) under study. Overall, better understanding of the effects immobilization chemistries have on cell adhesion peptide activity may lead to more efficacious coatings for biomedical devices.
Coatings, 2020. Link here |
|
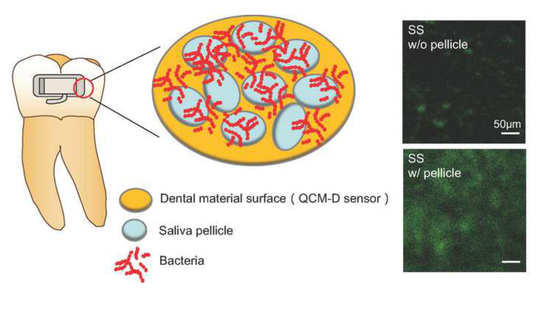
Dental materials are susceptible to dental plaque formation, which increases the risk of biofilm associated oral diseases. Physical-chemical properties of dental material surfaces can affect salivary pellicle formation and bacteria attachment, but relationships between these properties have been understudied. We aimed to assess the effects of surface properties and adsorbed salivary pellicle on Streptococcus gordonii adhesion to traditional dental materials. Adsorption of salivary pellicle on gold, stainless steel, alumina and zirconia was monitored with a quartz crystal microbalance with dissipation monitoring (QCM-D). Our findings suggested that the critical factor increasing S. gordonii attachment was the salivary pellicle formed on dental materials. This is attributed to increased work of adhesion between bacteria and substrates with pellicle. New dental materials should be designed for controlling bacteria attachment by tuning thickness, composition and structure of the adsorbed salivary pellicle.
Colloids and Surfaces B: Biointerfaces, 2020. Link here |
|
The successful process of transcutaneous metal integration into host bone requires three synergistic systems: the host bone, the metal implant, and the skin-implant interface. All three systems must be optimized for successful incorporation and longevity of the implant. Osseointegration begins during surgical implantation of the metal components through a complex interplay of cellular mechanisms. While implants can vary in design – including the original screw, press fit implants, and compressive osseointegration - they face common challenges to successful integration and maintenance of fixation within the host bone. Overcoming these challenges requires the understanding of the complex interactions between each element of OI. This review outlines 1) the basic components of OI, 2) the science behind both the bone-implant and the skin-implant interfaces, 3) the current challenges of OI, and 4) future opportunities within the field.
Journal of Orthopedic Research, 2019. Link here |
|
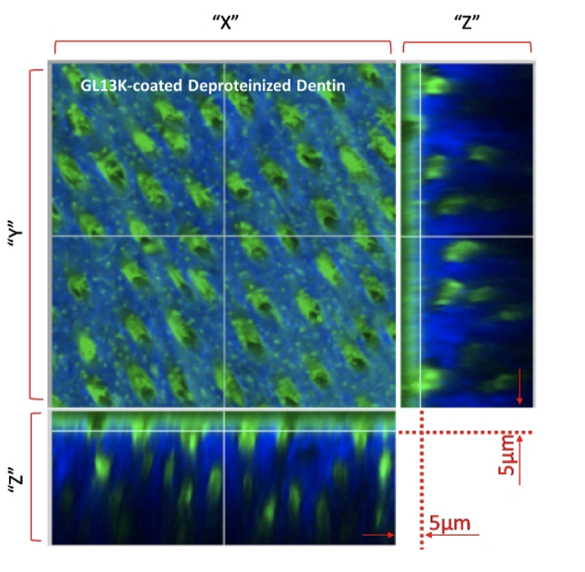
The evolution of bonded restorations has undergone a great progress over several decades. Over time, water and waterborne agents (acids, enzymes) degrade the components of the dentin/restoration interface allowing bacterial colonization and dentin re-infection at the margins of the restoration. We developed a 2- tier protective technology consisting of priming/coating dentin with amphipathic and antimicrobial peptides (AAMPs) to obtain hydrophobic/water-repellent and antibiofilm dentin resisting recurrent caries around bonded restorations. We tested a series of AAMPs to assess their structure-function relationships as well as the effects of different dentin-conditioning methods on the structural features of AAMPs-coated dentin. We also determined that AAMPs had preferential adsorption on the mineral phase of dentin, which suggested that peptides arrange their cationic and hydrophilic motifs in direct contact with the negatively-charged minerals in the hydrophilic dentin. We innovatively imaged the spatial distribution of the AAMPs in relation to the dentinal tubules and collagen network using a minimally invasive multimodal imaging technique, multi photon-second harmonic generation (MP- SHG). Using MP-SHG, we determined that partial deproteinization of dentin increased the amount of immobilized AAMPs compared to the total-etched dentin at the dentin surface and extended deeply around dentinal tubules. In conclusion, priming dentin with AAMPs is a versatile new approach with potential to fortify the otherwise vulnerable adhesive-based interfaces.
Journal of Dental Research, 2019. Link here |